Functionalization of diazotetronic acid and application in a stereoselective modular synthesis of pulvinone, aspulvinones A–E, G, Q and their analogues†
Abstract
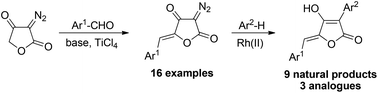
A modular synthesis of aspulvinones A, B, C, D, E, G and the recently isolated aspulvinone Q was developed. The methodology features a highly stereoselective aldol condensation of diazotetronic acid with aldehydes to provide 5-arylidene diazotetronates. Subsequent catalytic intermolecular C–H insertion reactions of the arylidene tetronates with arenes provide a series of naturally occurring aspulvinones including aspulvinones C, D and Q which have not been synthesized before. Variation of the aldehyde and the arene components furnishes synthetic analogues of the aspulvinones.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...