Rhodium-catalyzed cyclization of acceptor-substituted biphenyl α-diazoketones: a study of the substitution effect on chemoselectivity†
Abstract
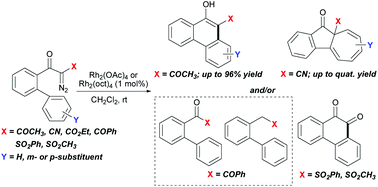
A range of biphenyl α-diazoketones containing various electron-withdrawing groups (EWG = COCH3, CN, CO2Et, COPh, SO2CH3, SO2Ph) on diazo carbon has been investigated for rhodium(II)-catalyzed intramolecular cyclization. Among which, the α-acetyl, carboxylate and cyano substituted substrates show markedly different selectivity between aromatic substitution and aromatic cycloaddition processes, affording phenanthrol and/or benz[α]azulenone products in varying ratios. The selectivity is mainly directed by α-substitutions, and is also possibly influenced by the substituents on the biphenyl ring. Moreover, high chemoselectivity for aromatic substitution over cycloaddition is observed for the α-benzoyl and sulfonyl substituted substrates. But in addition to phenanthrols, these reactions produce aromatic ketones and/or 1,2-diketones as unprecedented products obtained from diazo precursors. Mechanistic rationales are given in the report.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...