Preparation of oxazolines and oxazoles via a PhI(OAc)2-promoted cyclization of N-propargylamides†
Abstract
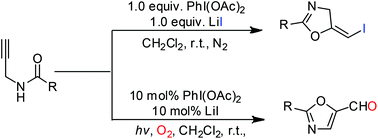
A metal-free cyclization of N-propargylamides for the synthesis of various oxazolines and oxazoles via a 5-exo-dig process is presented. Using (diacetoxyiodo)benzene (PIDA) as a reaction promoter and lithium iodide (LiI) as an iodine source, intramolecular iodooxygenation of N-propargylamides proceeded readily, leading to the corresponding (E)-5-iodomethylene-2-oxazolines in good to excellent isolated yields. In addition, using the PhI(OAc)2/LiI system, N-propargylamides can be converted to the corresponding oxazole-5-carbaldehydes in the presence of oxygen under visible light irradiation. The resulting products can be further converted into various oxazoline and oxazole derivatives after simple derivatizations, and this method ultimately offers an efficient route to a variety of biologically active structures.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...