Elucidation of the catalytic mechanism of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase using QM/MM calculations†
Abstract
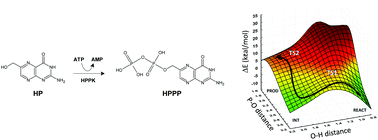
The folate pathway is a recognized intervention point for treating parasitic and bacterial infections in humans. However, the efficacy of treatments targeting dihydropteroate synthase (DHPS) and dihydrofolate reductase (DHFR) has reduced due to disease-related mutations. This has prompted interest in other enzyme targets on this clinically validated pathway, including 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase (HPPK). A challenge in the design of molecules to target this enzyme is that the precise mechanism of the reaction and the role of the active site residues are not fully understood. In this study, we report the first theoretical analysis of the catalytic pathway of the natural substrate using hybrid quantum mechanical/molecular mechanical (QM/MM) methods. The reaction profiles associated with three proposed general bases have been investigated, as well as the profile for two mutant enzymes, namely R92A and R82A. We identified R92 as the general base in the wildtype reaction. The predicted barriers are in good agreement with the observed experimental kcat values obtained for wildtype and mutant proteins.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...