Indium(iii) as π-acid catalyst for the electrophilic activation of carbon–carbon unsaturated systems
Abstract
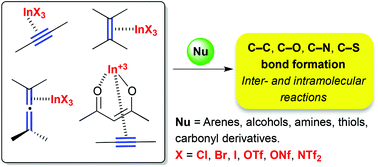
This review focuses on the utilization of indium(III) as π-acid for the activation of C–C unsaturated bonds in organic synthesis. In addition to its well-known σ-coordination with carbonyl derivatives, indium(III) undergoes efficient π-coordination with unsaturated systems to trigger nucleophilic addition. Accordingly, indium(III) halides and salts (InX3, X = Cl, Br, I, OTf, ONf, NTf2) have been reported as useful catalysts for a broad range of carbon–carbon and carbon–heteroatom bond formation reactions, including hydrofunctionalization (hydroarylation, hydroamination, hydroalkoxylation, and hydrothiolation), enyne cycloisomerization, and related reactions. In these reactions the counterion has a significant effect on the catalytic activity, and the development of novel In(III) complexes and the generation of highly electrophilic cationic indium(III) species has increased its synthetic applications as a π-acid catalyst.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...