Recent developments in functionalization of acyclic α-keto amides
Abstract
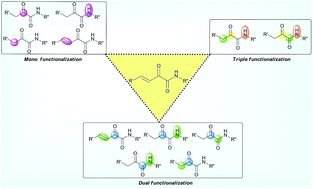
α-Keto amides are a key frame in biology, medicinal chemistry and synthetic chemistry as drug molecules and key intermediates. Based on the structural aspect, an acyclic α-keto amide has multiple reactive centres which have electrophilic and nucleophilic characters. In this review, we focus on the recent developments of both asymmetric and achiral reactions involving acyclic α-keto amides as the reactants for the synthesis of cyclic and acyclic molecules through the formation of C–C, C–O, C–N and C–H bonds. Based on the reactivity of α-keto amides, this article is divided into three major classes: 1. mono-functionalization, 2. dual functionalization, and 3. triple functionalization of α-keto amides, which are further divided into sub-classes as well. Over 50 examples including our own work have been demonstrated for the functionalization of acyclic α-keto amides since 1992. Moreover, the usefulness of α-keto amides is shown for the synthesis of biologically active molecules, synthetic intermediates, heterocycles, valuable compounds, building blocks and metal complexes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...