Pd(ii)-Catalyzed aerobic 1,2-difunctionalization of conjugated dienes: efficient synthesis of morpholines and 2-morpholones†
Abstract
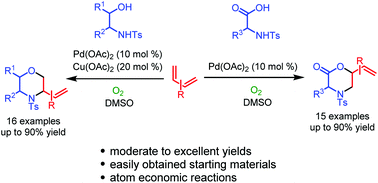
A novel and efficient methodology concerning the Pd(II)-catalyzed intermolecular difunctionalization of conjugated dienes is reported to synthesize a series of functionalized morpholines and 2-morpholones. Widely distributed and easily obtained β-amino alcohols and α-amino acids, as starting nitrogen and oxygen sources, are successfully applied in the difunctionalization of conjugated dienes respectively. The majority of the desired products were obtained in moderate to excellent yields. Oxygen was successfully employed as a terminal oxidant. Further transformation of the generated products allowed for the expansion of structural diversity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...