Enantioselective total synthesis and biological evaluation of (−)-solanacol†
Abstract
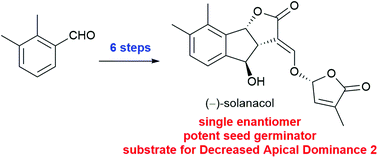
An enantioselective synthesis of the phenyl ring-containing strioglactone, (−)-solanocol, is described. Application of a Dynamic Kinetic Resolution (DKR) in the stereo-defining step enabled a step-economical synthesis to be achieved, and allowed access to natural and non-natural enantiomers with equal facility. Results of seed germination assays and Differential Scanning Fluorimetry (DSF) measurements with the known strigolactone receptor protein, Decreased Apical Dominance 2 (DAD2), are reported.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...