Enantioselective synthesis of α-perfluoroalkylated prolines, their 6,7-membered homologues and derivatives†
Abstract
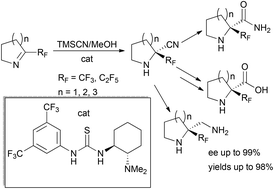
Enantioselective synthesis of α-perfluoroalkylated cyclic amino acids and their derivatives was elaborated using the organocatalytic Strecker reaction with 5–7 membered cyclic ketimines. The prepared amino nitriles could be transformed into chiral Rf-prolines and their 6,7-membered homologues as well as their corresponding amides and diamines (yields up to 99%, up to >99% ee).
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...