Inexpensive NaX (X = I, Br, Cl) as a halogen donor in the practical Ag/Cu-mediated decarboxylative halogenation of aryl carboxylic acids under aerobic conditions†
Abstract
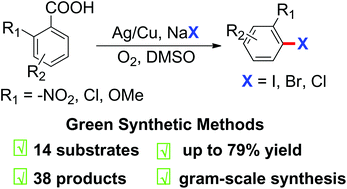
Versatile and practical Ag/Cu-mediated decarboxylative halogenation between readily available aryl carboxylic acids and abundant NaX (X = I, Br, Cl) has been achieved under aerobic conditions in moderate to good yields. The halodecarboxylation is shown to be an effective strategy for S-containing heteroaromatic carboxylic acid and benzoic acids with nitro, chloro and methoxyl substituents at the ortho position. A gram-scale reaction and a three-step procedure to synthesize iniparib have been performed to evaluate the practicality of this protocol. A preliminary mechanistic investigation indicates that Cu plays a vital role and a radical pathway is involved in the transformation.


 Please wait while we load your content...
Please wait while we load your content...