Silver-catalyzed regioselective hydroamination of alkenyl diazoacetates to synthesize γ-amino acid equivalents†
Abstract
A simple protocol to directly access γ-amino acid derivatives by intermolecular regioselective hydroamination of trichloroethyl alkenyldiazoacetates with carbamate using a silver tetrafluoroborate catalyst is described. Density functional theory (DFT) calculations to analyze the reaction mechanism revealed that multiple attractive interactions occur in a transition state to promote the vinylogous addition of nitrogen nucleophiles.
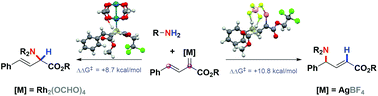
- This article is part of the themed collection: Carbenes in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...