TsOH·H2O-mediated N-amidation of quinoline N-oxides: facile and regioselective synthesis of N-(quinolin-2-yl)amides†
Abstract
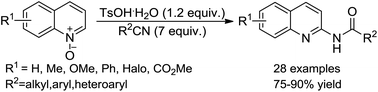
An operationally simple method with 100% atom economy has been developed for the synthesis of various N-(quinolin-2-yl)amides via the TsOH·H2O-mediated N-amidation of quinoline N-oxides using inexpensive and commercially available nitriles as the amidation reagents. Mechanistic exploration suggested that the reaction probably proceeds through an acid-assisted 1,3-dipolar cycloaddition and an N–O bond cleavage followed by a dehydro-aromatization process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...