Studies on the interactions of 5-R-3-(2-pyridyl)-1,2,4-triazines with arynes: inverse demand aza-Diels–Alder reaction versus aryne-mediated domino process†
Abstract
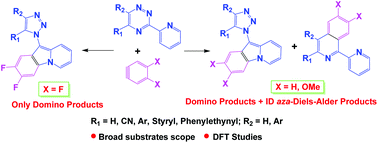
The interactions between substituted 5-R-3-(pyridyl-2)-1,2,4-triazines with in situ generated substituted aryne intermediates have been studied. The reaction afforded either inverse demand (ID) aza-Diels–Alder products or 1,2,4-triazine ring rearrangement (domino) products as major ones depending on the nature of both the substituents at the C5 position of the 1,2,4-triazine core or in the aryne moiety. The structures of the key products were confirmed based on X-ray data. Based on the density functional theoretical (DFT) studies of the Diels–Alder transition state geometries, the influence of the nature of arynes on the direction of the 1,2,4-triazine transformation has been proposed.


 Please wait while we load your content...
Please wait while we load your content...