An ab initio and DFT study of trifluoromethylation using Umemoto's reagent†
Abstract
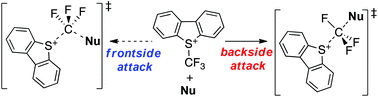
Trifluoromethylation using Umemoto's reagent is an important transformation that allows the preparation of compounds bearing trifluoromethyl groups. To investigate the mechanism of this reaction, ab initio and density functional theory (DFT) calculations were carried out using pyrrole, aniline, sodium acetylacetonate, and sodium methyl acetoacetate as nucleophiles. At the highest level of theory examined (i.e., CCSD(T)/6-311+G(d,p)//M06-2X/6-311+G(d,p)), the energy barriers for the forward process (ΔE‡1) of both the backside and frontside attack of pyrrole on a model Umemoto reagent (i.e., S-(trifluoromethyl)dimethylsulfonium, CF3DMS) were predicted to be 135.9 and 192.3 kJ mol−1, respectively, while values of 131.9 and 188.2 kJ mol−1 were obtained at the MP2/6-311+G(d,p)//M06-2X/6-31+G(d,p) level. These outcomes suggest that the reaction proceeds via the backside mechanism. Using the MP2 method, the investigation of the trifluoromethylation of pyrrole and sodium acetoacetate with the sulfonium moiety of Umemoto's reagent, S-(trifluoromethyl)dibenzothionium, revealed that this reaction would also occur through the backside mechanism, thereby indicating that this pathway remains feasible despite solvent effects. Finally, computational investigations revealed that the simple single-electron transfer mechanism, which should occur between Umemoto's reagent and nucleophiles, did not take place during this reaction.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...