Rhodium(iii)-catalyzed CF3-carbenoid C–H functionalization of 6-arylpurines†
Abstract
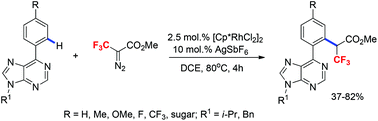
An efficient method for the CF3-carbenoid C–H functionalization of 6-arylpurines has been developed. This protocol uses readily available methyl 3,3,3-trifluoro-2-diazopropionate as a cross-coupling partner and proceeds smoothly under chelation-controlled Rh(III) catalysis. The reactions provide the corresponding carbene insertion products with high regioselectivity within a few hours and allow the introduction of both the CF3 and carboxylate functions into biologically important purine molecules including nucleoside derivatives.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...