Synthesis of topologically constrained naphthalimide appended palladium(ii)–N-heterocyclic carbene complexes – insights into additive controlled product selectivity†
Abstract
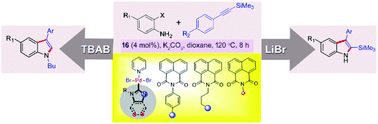
Topologically constrained naphthalimide appended Pd–NHCs were synthesized and characterized. These structurally related complexes were catalytically compared with previously synthesized Pd–NHCs in the regioselective heteroannulation of o-haloanilines and arylethynyl-trimethylsilane. The unique effect of an additive on product selectivity has been clearly demonstrated. The scope of the reaction with respect to different TMS protected alkynes and o-haloanilines is presented. Importantly, the step-economical regioselective synthesis of N-alkyl-3-aryl-indoles from o-haloanilines and arylethynyl-trimethylsilane assisted by Pd(II)–NHCs has been clearly demonstrated via one-pot heteroannulation, TMS deprotection and N-alkylation. In addition, synthetic utility was demonstrated with several derivatizations.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...