Gold(i)- and rhodium(iii)-catalyzed formal regiodivergent C–H alkynylation of 1-arylpyrazolones†
Abstract
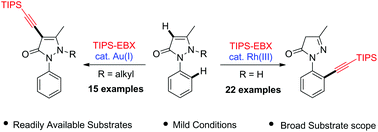
Formal regiodivergent C–H alkynylation of 1-aryl-5-pyrazolones has been realized under the catalysis of Rh(III) and Au(I) complexes by using a hypervalent iodine reagent as the alkyne source. Mechanistic studies indicate that the regioselectivity is ascribed to not only the choice of the catalyst but also the nature of the substrate. The substrate scope and functional group compatibility have been fully examined.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...