An asymmetric Mannich reaction of α-diazocarbonyl compounds and N-sulfonyl cyclic ketimines catalyzed by complexes generated from chiral and achiral phosphines with gold(i)†
Abstract
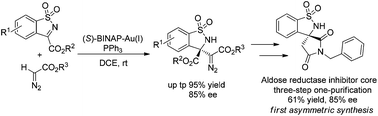
An unprecedented Lewis acidic gold(I)-complex generated from chiral and achiral phosphines has been developed for the Mannich reaction of α-diazocarbonyl compounds and N-sulfonyl cyclic ketimines. A series of chiral β-amino-α-diazoesters bearing a quaternary stereocenter were obtained in high yields with good enantioselectivities. In addition, the products could be converted to promising bioactive spirosuccinimide. Furthermore, operando IR, NMR and control experiments were carried out to gain insight into the mechanism.


 Please wait while we load your content...
Please wait while we load your content...