Total synthesis of (±)-tanshinol B, tanshinone I, and (±)-tanshindiol B and C†
Abstract
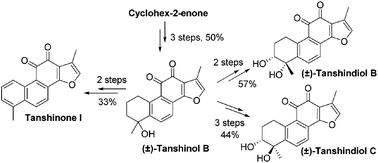
A concise and efficient approach was established for the divergent total synthesis of (±)-tanshindiol B and C and tanshinone I from a ubiquitous ene intermediate in 1–2 steps. This critical intermediate was derived from (±)-tanshinol B, which was synthesized in 50% overall yield over 3 steps using an ultrasound-promoted cycloaddition as a key step. Compared to a previously reported strategy, our approach is more step-economic, thus greatly improving the synthetic efficiency. The bioactivity evaluation indicates that the diol stereochemistry of the tanshidiols has an impact on the EZH2 inhibitory activity.


 Please wait while we load your content...
Please wait while we load your content...