α-Methyl phenylglycines by asymmetric α-arylation of alanine and their effect on the conformational preference of helical Aib foldamers†
Abstract
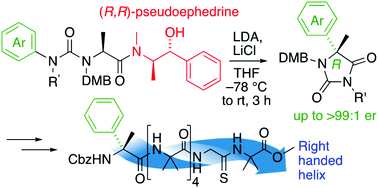
α-Arylated alanine derivatives were made enantioselectively by migratory rearrangement of a urea derivative using (R,R)-pseudoephedrine as a chiral auxiliary. Incorporation of a single residue of the product α-methyl phenylglycine into an otherwise achiral oligomer of aminoisobutyric acid oligomer induced a preferred screw sense, detectable by a NMR reporter located at the remote terminus of the oligomer. The magnitude of the screw sense induction was greater when the chiral residue was located at the N-terminus of the foldamer, and in some cases the sense of induction was opposite to that of related α-methylated amino acids with α-substituents other than aryl.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...