Iron-catalyzed ortho trifluoromethylation of anilines via picolinamide assisted photoinduced C–H functionalization†
Abstract
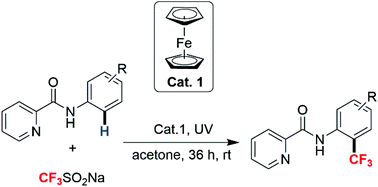
A convenient, oxidant-free protocol was developed for the ortho trifluoromethylation of aniline via picolinamide assisted Fe-promoted C–H functionalization under ultraviolet irradiation. In this transformation acetone essentially acted as both a solvent to dissolve reactants and a low-cost radical initiator to efficiently generate a CF3 radical from Langlois’ reagent. A broad substrate scope was tolerated and picolinamide bearing strong electron withdrawing groups also could be transformed into the corresponding products with acceptable yields. Furthermore, the value of this method has been highlighted via the efficient synthesis of the nonsteroidal anti-inflammatory drug floctafenine.


 Please wait while we load your content...
Please wait while we load your content...