Glycosyl nitrates in synthesis: streamlined access to glucopyranose building blocks differentiated at C-2†
Abstract
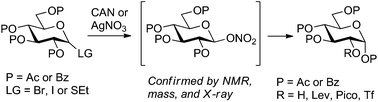
In an attempt to refine a CAN-mediated synthesis of 1,3,4,6-tetra-O-acetyl-α-D-glucopyranose (2-OH glucose) we unexpectedly discovered that this reaction proceeds via the intermediacy of glycosyl nitrates. Improved mechanistic understanding of this reaction led to the development of a more versatile synthesis of 2-OH glucose from a variety of precursors. Also demonstrated is the conversion of 2-OH glucose into a variety of building blocks differentially protected at C-2, a position that is generally hard to protect regioselectively in the glucopyranose series.


 Please wait while we load your content...
Please wait while we load your content...