Concerning the mechanism of iodine(iii)-mediated oxidative dearomatization of phenols†
Abstract
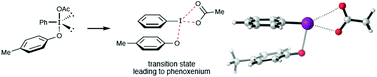
The iodine(III)-mediated oxidative dearomatization of phenols has proven to be a general method for the preparation of cyclohexadienones. While this is a widely used reaction, there is still a great deal of uncertainty regarding the mechanistic pathway followed by these reactions. In part, this is due to the highly unstable nature of many of the key intermediates, which makes their direct detection extremely difficult. In order to gain some insight into these mechanistic questions, DFT calculations [M06-2X/6-31+G(d) for C, H, and O and LANL2DZdp for iodine] were used to evaluate the two most commonly proposed reaction mechanisms. These results show that unimolecular fragmentation of an oxygen-bound intermediate to give a phenoxenium ion (TS1) is preferred over direct addition of the nucleophile to the aromatic ring of the activated phenol (TS3). In addition, results are presented that suggest protonation and/or hydrogen bonding may play a key role in lowering the energy of the unimolecular fragmentation pathway.

 Please wait while we load your content...
Please wait while we load your content...