Efficient syntheses and anti-cancer activity of xenortides A–D including ent/epi-stereoisomers†
Abstract
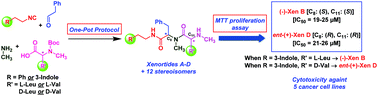
A one-pot, two-step, total synthesis of naturally occurring xenortides A, B, C and D, (Xens A–D) isolated from the bacterium Xenorhabdus nematophila, and an entire complementary set of stereoisomers, has been achieved. Compounds were synthesized utilizing an isocyanide-based Ugi 4-CR followed by facile N-Boc deprotection. The reaction sequence took advantage of the chiral pool of N-Boc protected amino acids (L-Leu/Val and D-Leu/Val) with aryl isocyanides, phenyl acetaldehyde and methylamine giving the desired Xens A–D (A and B >98% ee) and all subsequent stereoisomers in reasonable yields upon deprotection followed by separation of diastereomers. Also, detailed mechanistic insights for diastereoselectivity of (−)-Xen A, as a model in the Ugi 4-CR, has been described. Moreover, for the first time, this focused library was screened for cytotoxicity against a panel of epithelial cancer cell lines as well as normal cell lines with an MTT proliferation assay. The structure–activity relationship (SAR) study demonstrated that tryptamides Xen B and D were more active than phenylethylamides Xen A and C. Furthermore, (−)-Xen B (IC50 = 19–25 μM) and ent-(+)-Xen D (IC50 = 21–26 μM) gave the highest cytotoxicity and they were also found to be non-toxic toward normal cells. Importantly, the SAR results indicate that the stereochemistry at C8 and C11 in (−)-Xen B and ent-(+)-Xen D play a critical role in cytotoxic activity.


 Please wait while we load your content...
Please wait while we load your content...