Gold(i)-catalyzed diastereoselective synthesis of 1-α-oxybenzyl-1H-indenes†
Abstract
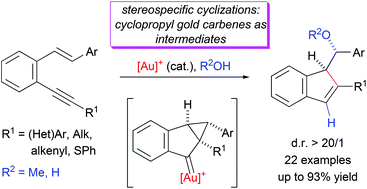
The gold(I)-catalyzed oxycyclization of β-aryl monosubstituted o-(alkynyl)styrenes gives rise to 1-α-methoxy or 1-α-hydroxybenzyl-1H-indenes in a diastereospecific way. In contrast to β,β-disubstituted o-(alkynyl)styrenes, the stereochemical outcome of this process, diastereospecific reaction supports the higher contribution of a gold intermediate with a cyclopropylcarbene-like character.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...