Bismuth(iii)-catalyzed cycloisomerization and (hetero)arylation of alkynols: simple access to 2-(hetero)aryl tetrahydrofurans and tetrahydropyrans†
Abstract
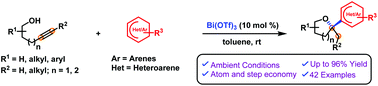
2-(Hetero)aryl tetrahydrofurans and tetrahydropyrans were successfully synthesized using Bi(OTf)3-catalyzed hydroalkoxylation (cycloisomerization) of alkynols (via 5 or 6 exo-dig cyclization) and intermolecular (hetero)arylation. This reaction involves a highly efficient cascade process, where initially the alkynol undergoes a cycloisomerization step via activation of the triple bond and generates the oxocarbenium ion, which subsequently participates in the (hetero)hydroarylation step with electron-rich arenes. Simple to complex suitably functionalized alkynols (4-pentyn-1-ols and 5-hexyn-1-ols) and electron-rich aromatic compounds were found to be reliable substrates in this cascade transformation and furnished a wide range of oxygen heterocycles. This practical tandem process provides a means to build libraries related to pharmacologically active molecules and natural product like scaffolds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...