C5-Morpholinomethylation of N1-sulfonylcytosines by a one-pot microwave assisted Mannich reaction†
Abstract
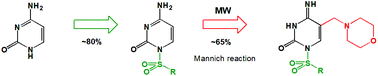
A fast and efficient route for the introduction of a methylene bridged-amine (morpholinomethyl) functionality in the C5 position of the sulfonylated cytosine nucleobase has been developed. First, novel N1-sulfonylcytosine derivatives 3–6 were prepared by the condensation of silylated cytosine with selected sulfonyl chlorides. They were subsequently transformed to 5-morpholinomethyl-N1-sulfonylcytosine derivatives (8, 12–15) using microwave irradiation. As a result of cytosine ring opening in N1-tosylcytosine, depending on the reaction conditions, peculiar tosyl-urea derivative 9 has been isolated, which provided additional insight into the reaction pathway. The influence of the C5-substituent on the antiproliferative activity has been evaluated by performing the MTT test on U251, MCF-7 and MOLT-4 tumor cell-lines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...