Pd-catalyzed intramolecular C(sp2)–H amination of phenylalanine moieties in dipeptides: synthesis of indoline-2-carboxylate-containing dipeptides†
Abstract
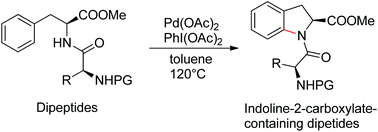
A palladium-catalyzed intramolecular C(sp2)–H amination of phenylalanine moieties in dipeptides is described. By this protocol, a series of indoline-2-carboxylate-containing dipeptides were synthesized from dipeptides. The N-protected amino acid moiety within the peptide is used as an intrinsic bidentate directing group. This intramolecular C–H amination reaction provides an appealing strategy for the post-synthetic modification of peptides.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...