Synthesis of highly functionalized 1,6-dihydropyridines via the Zn(OTf)2-catalyzed three-component cascade reaction of aldimines and two alkynes (IA2-coupling)†
Abstract
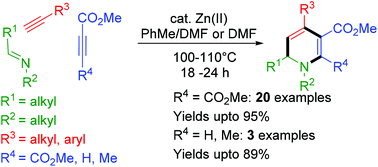
A zinc(II) triflate catalyzed three component synthesis of 1,6-dihydropyridines, involving aldimines, alkynes and electron-deficient dimethyl acetylenedicarboxylate (DMAD), in good to excellent yields has been described. Besides a range of different N-substituents, a variety of both aromatic and aliphatic alkynes could be used. The application of electron-deficient propiolates instead of DMAD resulted in regiospecific incorporation of the ester functionality on the 1,6-dihydropyridine ring. The reaction proceeds via a cascade reaction involving nucleophilic addition of the metal acetylide to the imine, followed by the addition of the intermediately formed propargylic amine to the electron-deficient alkyne and subsequent 6-endo dig cyclization.


 Please wait while we load your content...
Please wait while we load your content...