Palladium-catalyzed dimerization of N-aryl propargylamines for the synthesis of 3-vinylquinolines†
Abstract
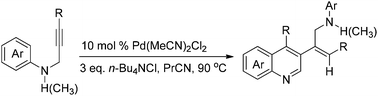
A straightforward method for the synthesis of polyfunctionalized quinolines from readily available N-aryl propargylamines under aerobic conditions was developed. It provides convenient access to a variety of synthetically and pharmaceutically important quinolines in moderate to good yields. Control experiments suggest that the cascade reaction might proceed via the Pd-catalyzed electrophilic cyclization of N-aryl propargylamines followed by a hydroarylation process through trapping of the σ-quinolinylpalladium intermediate with a second molecule of the substrate.


 Please wait while we load your content...
Please wait while we load your content...