Access to 2-substituted-2H-indazoles via a copper-catalyzed regioselective cross-coupling reaction†
Abstract
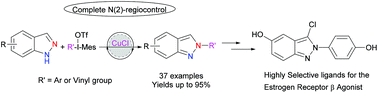
A CuCl catalyzed C–N cross-coupling reaction using commercially available 1H-indazoles with diaryliodonium salts is described. The methodology features ample structural versatility, affording 2-substituted-2H-indazole in good yields and complete N(2)-regiocontrol. Furthermore, the utility of the reaction was demonstrated in the synthesis of a known estrogen receptor β agonist. Mechanistic studies using density functional theory calculations suggested that the complete regioselectivity can be attributed to the only weak base TfO− in our system which could not deprotonate indazoles, and the catalyst oxidation process would be the rate-determining step.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...