KI-catalyzed C–S bond formation via an oxidation relay strategy: efficient access to various α-thio-β-dicarbonyl compounds†
Abstract
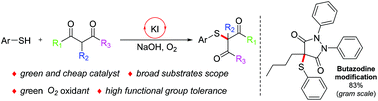
An efficient and practical methodology to obtain α-thio-β-dicarbonyl compounds was presented under alkaline conditions via potassium iodide (KI) catalysis; various symmetrical/unsymmetrical 1,3-dicarbonyl compounds were obtained under an aerobic atmosphere in moderate to excellent yields, with good functional group tolerance. Notably, a widely used anti-inflammatory drug butazodine could be modified with our protocol, even on a gram scale.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...