Organocatalytic asymmetric Michael/hemiacetalization/acyl transfer reaction of α-nitroketones with o-hydroxycinnamaldehydes: synthesis of 2,4-disubstituted chromans†
Abstract
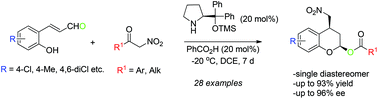
An organocatalytic asymmetric cascade Michael/hemiketalization/acyl transfer reaction between o-hydroxycinnamaldehydes and α-nitroketones is developed. Prolinol TMS ether catalyst in combination with benzoic acid was found to be the most effective for this reaction which proceeds through an equilibrium of lactols to provide a single diastereomer of enantiopure 2,4-disubstituted chromans.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...