QM/MM simulations identify the determinants of catalytic activity differences between type II dehydroquinase enzymes†
Abstract
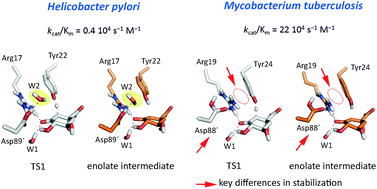
Type II dehydroquinase enzymes (DHQ2), recognized targets for antibiotic drug discovery, show significantly different activities dependent on the species: DHQ2 from Mycobacterium tuberculosis (MtDHQ2) and Helicobacter pylori (HpDHQ2) show a 50-fold difference in catalytic efficiency. Revealing the determinants of this activity difference is important for our understanding of biological catalysis and further offers the potential to contribute to tailoring specificity in drug design. Molecular dynamics simulations using a quantum mechanics/molecular mechanics potential, with correlated ab initio single point corrections, identify and quantify the subtle determinants of the experimentally observed difference in efficiency. The rate-determining step involves the formation of an enolate intermediate: more efficient stabilization of the enolate and transition state of the key step in MtDHQ2, mainly by the essential residues Tyr24 and Arg19, makes it more efficient than HpDHQ2. Further, a water molecule, which is absent in MtDHQ2 but involved in generation of the catalytic Tyr22 tyrosinate in HpDHQ2, was found to destabilize both the transition state and the enolate intermediate. The quantification of the contribution of key residues and water molecules in the rate-determining step of the mechanism also leads to improved understanding of higher potencies and specificity of known inhibitors, which should aid ongoing inhibitor design.


 Please wait while we load your content...
Please wait while we load your content...