A fluorescent 3,7-bis-(naphthalen-1-ylethynylated)-2′-deoxyadenosine analogue reports thymidine in complementary DNA by a large emission Stokes shift†
Abstract
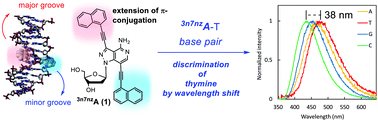
The new environmentally responsive fluorescent nucleosides, 3,7-bis-(naphthalen-1-ylethynyl)-8-aza-3,7-dideaza-2′-deoxyadenosine (3n7nzA, 1) and 7-(naphthalen-1-ylethynyl)-8-aza-3,7-dideaza-2′-deoxyadenosine (37nzA, 2), have been synthesized. Both 3n7nzA (1) and 37nzA (2) possess large π-conjugated systems which extend into both the minor and major grooves or the major groove alone, respectively. The nucleosides exhibited large solvatochromic shifts (3n7nzA: Δλ = 45 nm, 37nzA: Δλ = 78 nm) and were examined for their ability to fluorimetrically report hybridization events. When incorporated into ODN probes, the bis-substituted 3n7nzA (1) selectively recognized thymidine on target strands which was reported by a distinct change in its emission wavelength in the long wavelength region, whereas 37nzA (2) showed a preference for pairing to cytidine and a smaller wavelength shift. Thus, 3n7nzA (1) has the potential for use as a fluorescent probe for structural studies of DNAs/RNAs including the detection of single-base alterations in target DNA sequences.


 Please wait while we load your content...
Please wait while we load your content...