Thermal conversion of primary alcohols to disulfides via xanthate intermediates: an extension to the Chugaev elimination†
Abstract
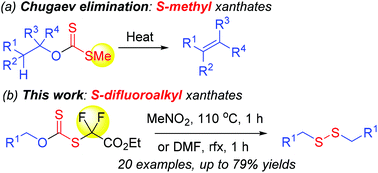
Primary alcohols are converted into dialkyl disulfides via heating in situ generated O-alkyl S-difluoro(ethoxycarbonyl)methyl xanthates from ethyl bromodifluoroacetate and potassium xanthates, prepared from primary alcohols and carbon disulfide in the presence of KOH. The reaction mechanism is suggested as an alkyl C[1,3] shift followed by a radical mechanism. This extends to the Chugaev elimination which yields olefins. The current research provides easy access to dialkyl disulfides from commercially available primary alkanols.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...