A metal-free and mild approach to 1,3,4-oxadiazol-2(3H)-ones via oxidative C–C bond cleavage using molecular oxygen†
Abstract
A mild metal-free approach to 1,3,4-oxadiazol-2(3H)-ones via 1,3,4-oxadiazin-5(6H)-ones is described. This novel transformation, promoted by the electron-withdrawing p-substituents on the phenyl group at the α-carbonyl position, features a tandem reaction consisting of oxidative hydroxylation and C–C bond cleavage using molecular oxygen. The method utilizes K2CO3 in CH3CN without any oxidants, transition metals, or additives, enabling the tunable synthesis of 1,3,4-oxadiazin-5(6H)-ones, 1,3,4-oxadiazol-2(3H)-ones, and α-ketoamides under mild aerobic conditions.
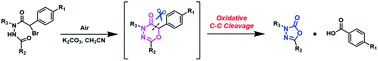
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...