Methods for the synthesis of annulated pyrroles via cyclisation strategies
Abstract
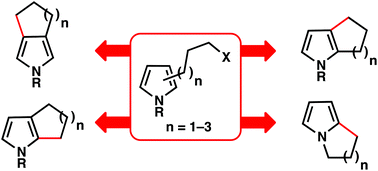
Pyrrole, pyrrolidine, and indolizidine alkaloids represent important classes of natural products. Historically, these heterocycles and their derivatives have been the focus of significant interest in organic synthesis. In this report, we review the methods that have been employed to synthesise annulated pyrroles via transformations that include: Friedel–Crafts acylations and alkylations, Michael additions, Heck couplings, hydroarylations, carbenoid insertions, and radical cyclisations.


 Please wait while we load your content...
Please wait while we load your content...