t-BuONa-mediated direct C–H halogenation of electron-deficient (hetero)arenes†
Abstract
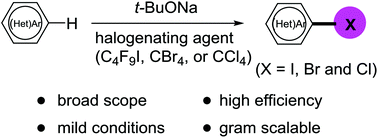
An efficient halogenation of electron-deficient (hetero)arenes is described. The reaction utilizes common t-BuONa as a catalyst (for iodination) or a promoter (for bromination and chlorination), and perfluorobutyl iodide, CBr4 or CCl4 as the readily-available halogenating agents, respectively. The protocol features broad scope, high efficiency, mild conditions and gram scalability. An ionic pathway involving halogen bond formation and halophilic attack is proposed. The utility of the resulting iodinated heteroarenes is demonstrated in visible light-mediated Caryl–Caryl cross-coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...