Chemoselective and stereospecific iodination of alkynes using sulfonium iodate(i) salt†
Abstract
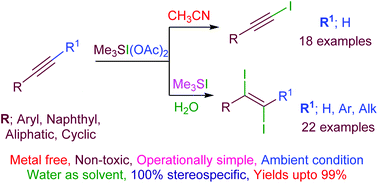
An efficient and highly chemoselective iodination of alkynes using a sulfonium iodate(I) electrophilc reagent under metal-free conditions has been realized. The reactivity of sulfonium iodate(I) salt could be significantly diverse in the presence of water as the solvent, enabling the (E)-1,2-diiodoalkenes stereospecifically. This stereodivergent approach is amenable to a wide range of alkyne substrates and demonstrates a diverse functional group tolerance resulting in synthetically valuable 1-iodoalkyne and (E)-vicinal-diiodoalkenes in good to excellent yields (up to 99%) with 100% selectivity under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...