Construction of the tetracyclic core of (±)-cycloclavine and 4-amino Uhle's ketone†
Abstract
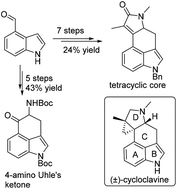
A rapid construction of the tetracyclic core (±)-2 of (±)-cycloclavine (1) was accomplished in seven steps and 24% overall yield from commercially available aldehyde 7. Key features include a domino Friedel–Crafts/nitro-Michael reaction to construct the C ring and an intramolecular ammonolysis of a diester to close the D ring. In addition, a versatile 4-amino Uhle's ketone (±)-3 was afforded rapidly in five steps and 43% overall yield.


 Please wait while we load your content...
Please wait while we load your content...