Highly chemoselective intermolecular cross-benzoin reactions using an ad hoc designed novel N-heterocyclic carbene catalyst†
Abstract
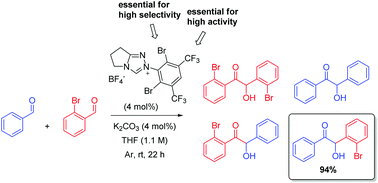
The design of a novel N-heterocyclic carbene catalyst incorporating a bulky yet highly electron-deficient N-aryl substituent has allowed the development of an efficient protocol for the first highly chemoselective intermolecular benzoin condensations between two non-identical aromatic aldehydes.


 Please wait while we load your content...
Please wait while we load your content...