Synthesis of unnatural α-amino acid derivatives via selective o-C–H functionalization†
Abstract
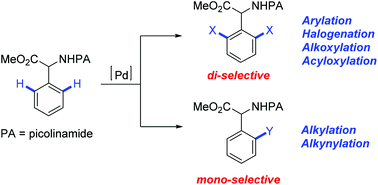
Pd-Catalyzed o-C–H functionalization of α-phenylglycine, 4-hydroxyphenylglycine and phenylalanine using picolinamide as a directing group is reported. We have developed different protocols for the arylation, alkylation, alkynylation, halogenation, alkoxylation, and acyloxylation of these amino acids. The reactions exhibit high selectivity, broad substrate scope, and compatibility with different functional groups in moderate to high yields. They provide a rapid and efficient access to a variety of phenyl based amino acid derivatives which can be further modified and have broad spectrum of applications in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...