Central-to-axial chirality induction in biphenyl chiroptical probes for the stereochemical characterization of chiral primary amines†‡
Abstract
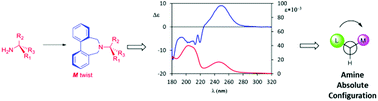
Flexible biphenyls have been applied as efficient and practical chiroptical probes for absolute configuration assignment to chiral primary amines. The mechanism of the central-to-axial chirality transfer from the amine moiety to the conformationally flexible biphenyl system has been determined by NMR and computational studies. This allowed proposing a general non-empirical rule in order to establish, simply by looking at the sign of the 250 nm A band in the ECD spectrum of the biphenyl derivative, the torsion of the biphenyl and thus the absolute configuration of the amine. The method proved to be very reliable and sensitive, allowing treatment of samples on the μmol scale and permitting the simultaneous determination of the amine sample's absolute configuration and enantiopurity.


 Please wait while we load your content...
Please wait while we load your content...