Narcissistic chiral self-sorting of molecular face-rotating polyhedra†
Abstract
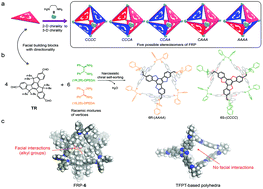
Narcissistic chiral self-sorting prevailed in the assembly of molecular face-rotating polyhedra from a C3h building block 5,5,10,10,15,15-hexabutyl-truxene-2,7,12-tricarbaldehyde and racemic mixtures of 1,2-diamines. Out of 124 possible stereoisomers, a pair of racemic polyhedra dominated, wherein (1R,2R)-diamines were segregated in AAAA polyhedra and (1S,2S)-diamines in CCCC polyhedra. This chiral self-sorting process is regulated by facial non-covalent interactions in the polyhedra. In contrast, D3h facial building blocks 1,3,5-tris-(4-formyl-phenyl)triazine and racemic mixtures of 1,2-diamines assembled into polyhedra without facial interactions, and their assembly process did not undergo apparent chiral self-sorting.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...