Novel conformationally constrained 2′-C-methylribonucleosides: synthesis and incorporation into oligonucleotides†
Abstract
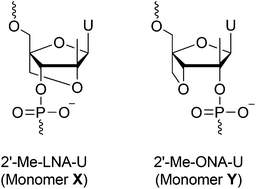
Synthesis of two novel conformationally constrained bicyclic ribonucleoside phosphoramidites bearing a 2′-C-methyl substituent has been accomplished. These phosphoramidites were used to incorporate the corresponding 2′-C-methyl nucleotides into oligonucleotides and to study their effects on duplex thermal stability. Whereas the C2′–O4′-linked LNA-type derivative induced severe destabilization of duplexes formed with complementary DNA and RNA, the C3′–O4′-linked derivative induced RNA-selective hybridization with increased affinity relative to that of the unmodified DNA-based probe.
- This article is part of the themed collection: Nucleic Acid Modifications


 Please wait while we load your content...
Please wait while we load your content...