Catalytic asymmetric enamine protonation reaction†
Abstract
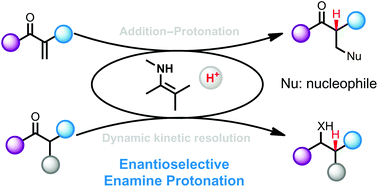
Enantioselective protonation, delivery of a proton to a carbanion intermediate, is the most straightforward and fundamental method for the preparation of a chiral tertiary carbon stereocenter. Recent efforts for this objective have been realized through enamine catalysis, which has now become a prominent catalytic strategy enabling a range of fascinating chiral transformations. This review will summarize recent advances in the field of enantioselective enamine protonation for the synthesis of optically active carbonyl compounds. Dynamic kinetic resolutions of α-substituted carbonyl compounds through enamine intermediates will be discussed as well.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...