The cinchona alkaloid squaramide catalyzed asymmetric Pictet–Spengler reaction and related theoretical studies†
Abstract
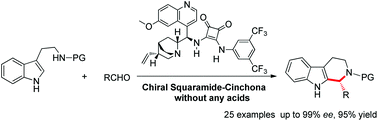
The asymmetric Pictet–Spengler reaction between tryptamine derivatives and aldehydes has been developed with a cinchona alkaloid squaramide as the catalyst, affording the corresponding tetrahedron-β-carbolines in good to excellent yields and ee values. A rational mechanistic pathway has also been proposed based on DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...