Thermally induced N–S bond insertion reaction of diazo compounds with N-sulfenylsuccinimides: synthesis of sulfides and mechanism studies†
Abstract
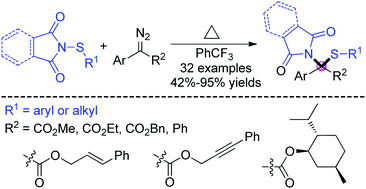
A metal-free gem-functionalization reaction of diazo compounds with N-sulfenylsuccinimides via a formal N–S bond insertion process has been reported. The transformation features mild reaction conditions, broad substrate scope and functional group tolerance, offering an efficient approach to sulfide synthesis in moderate to high yields. In addition, the mechanism studies indicate the formation of free carbene under thermal conditions followed by ylide generation, then N–S bond cleavage and C–N bond formation occurred in sequence to give the sulfide products.


 Please wait while we load your content...
Please wait while we load your content...