Tailoring the electrochemical activity of magnesium chromium oxide towards Mg batteries through control of size and crystal structure†
Abstract
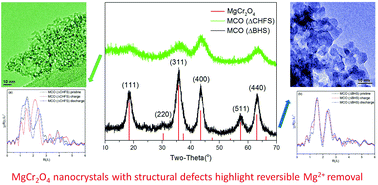
Chromium oxides with the spinel structure have been predicted to be promising high voltage cathode materials in magnesium batteries. Perennial challenges involving the mobility of Mg2+ and reaction kinetics can be circumvented by nano-sizing the materials in order to reduce diffusion distances, and by using elevated temperatures to overcome activation energy barriers. Herein, ordered 7 nm crystals of spinel-type MgCr2O4 were synthesized by a conventional batch hydrothermal method. In comparison, the relatively underexplored Continuous Hydrothermal Flow Synthesis (CHFS) method was used to make highly defective sub-5 nm MgCr2O4 crystals. When these materials were made into electrodes, they were shown to possess markedly different electrochemical behavior in a Mg2+ ionic liquid electrolyte, at moderate temperature (110 °C). The anodic activity of the ordered nanocrystals was attributed to surface reactions, most likely involving the electrolyte. In contrast, evidence was gathered regarding the reversible bulk deintercalation of Mg2+ from the nanocrystals made by CHFS. This work highlights the impact on electrochemical behavior of a precise control of size and crystal structure of MgCr2O4. It advances the understanding and design of new cathode materials for Mg-based batteries.
- This article is part of the themed collection: Nanoscale Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...
